These are the posts of the old forum. It was not possible to transfer the user data, so they are missing in most of the posts. For new questions, go to the general discussions.
Inverted curve - continued
- OldForum
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
14 years 5 months ago #1
by OldForum
Inverted curve - continued was created by OldForum
Please Log in or Create an account to join the conversation.
- OldForum
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
14 years 5 months ago #2
by OldForum
Replied by OldForum on topic Inverted curve - continued
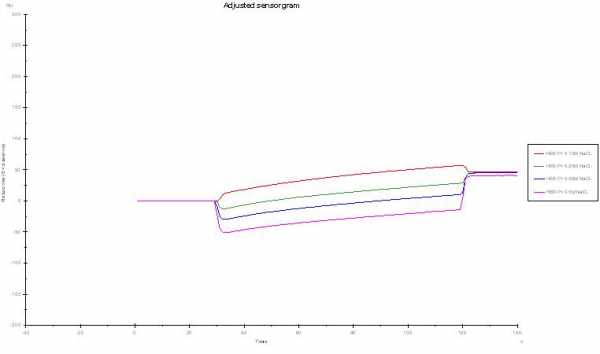
I see you use automatic reference subtraction. How are the raw curves? 0.5 M should give you a huge upwards jump.
Now you find -50 RU (0.5 M NaCl) after reference subtraction. It could be that the two channels react differently to the salt concentration due to different surface immobilization. It seems to me this is the case here, because the effect is concentration dependend.
Changing the FC. hmmm, Is system check alright? Is buffer injection allright? (pipet directly out the buffer bottle)--> there should be no differences in channels and after reference subtraction.
Arnoud
Now you find -50 RU (0.5 M NaCl) after reference subtraction. It could be that the two channels react differently to the salt concentration due to different surface immobilization. It seems to me this is the case here, because the effect is concentration dependend.
Changing the FC. hmmm, Is system check alright? Is buffer injection allright? (pipet directly out the buffer bottle)--> there should be no differences in channels and after reference subtraction.
Arnoud
Please Log in or Create an account to join the conversation.
- OldForum
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
14 years 5 months ago #3
by OldForum
Replied by OldForum on topic Inverted curve - continued
Please Log in or Create an account to join the conversation.
- OldForum
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
14 years 5 months ago #4
by OldForum
Replied by OldForum on topic Inverted curve - continued
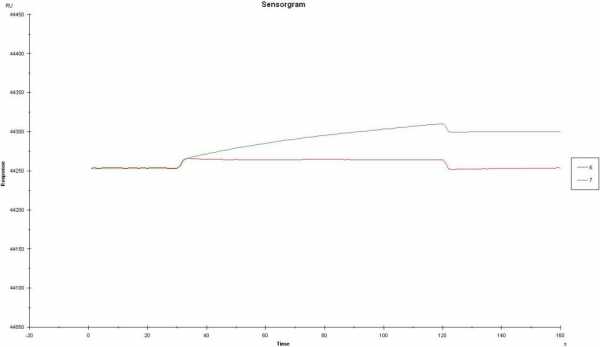
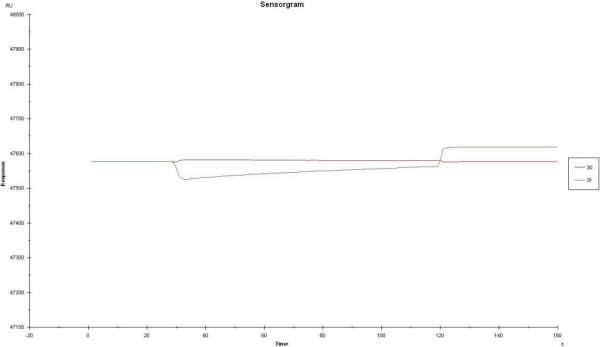
I am still a bit confused about which curve you subtract from which curve. If you inject the 0.5 M NaCl solution do you record two curves (1= with ligand and 2 = reference)?
Looking at curve 6 and 7 I should say this are not raw data since the buffer jump should be different between 0.5 and 0.15 M. Do you change the running buffer also to 0.15 and 0.5 M?
Do you inject salt only (no analyte)? Then you should not expect a binding curve but a buffer jump and flat line. You can use this curve for compensating differences in channel responses (double referencing).
The other thing is that running in 0.5 M NaCl will diminish binding of a lot of proteins. In fact is is a know recipe to dissociate proteins from sensor chips or columns.
Still confused whats going on.
Arnoud
Looking at curve 6 and 7 I should say this are not raw data since the buffer jump should be different between 0.5 and 0.15 M. Do you change the running buffer also to 0.15 and 0.5 M?
Do you inject salt only (no analyte)? Then you should not expect a binding curve but a buffer jump and flat line. You can use this curve for compensating differences in channel responses (double referencing).
The other thing is that running in 0.5 M NaCl will diminish binding of a lot of proteins. In fact is is a know recipe to dissociate proteins from sensor chips or columns.
Still confused whats going on.
Arnoud
Please Log in or Create an account to join the conversation.
- OldForum
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
14 years 5 months ago #5
by OldForum
Replied by OldForum on topic Inverted curve - continued
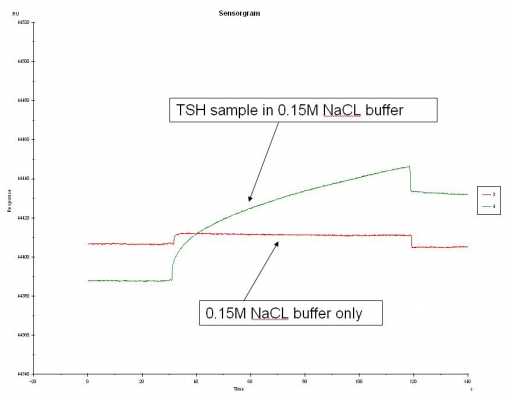
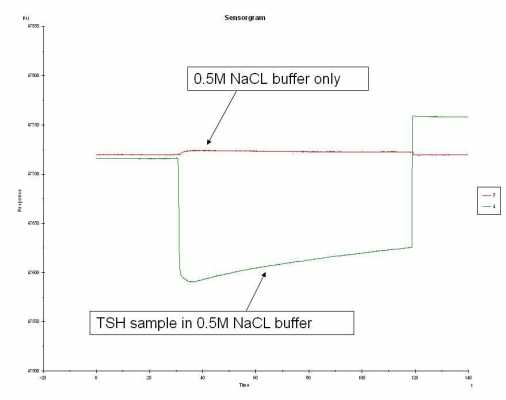
Sorry for the confusion. I did another simple experiment. Using FC 2, I injected the following: 1) 0.15M NaCL buffer only, 2) TSH in 0.15M NaCL, 3) 0.5M NaCL buffer only, and 4) TSH in 0.5M NaCL buffer. Each sample having each own cycle. I started with the 0.15M buffer and switched to the 0.5M buffer. The sensorgram below are labeled. Maybe try a diff. chip? running out of ideas...
Please Log in or Create an account to join the conversation.
- OldForum
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
14 years 5 months ago #6
by OldForum
Replied by OldForum on topic Inverted curve - continued
That is funny, you should expect with 0.5 M the same as with 0.15 M. I am also out of options why this happens and how to solve.
Try another chip or an other ligand analyte pair to make sure the machine is working properly.
Try another chip or an other ligand analyte pair to make sure the machine is working properly.
Please Log in or Create an account to join the conversation.