Sensor surface
The actual sensor chip surface depends on the manufacturer and the application. Generally, a glass surface is coated with an inert metal (gold). On the metal, a self-assembled monolayer (SAM) is deposited. This can be ω-hydroxyalkanethiol (1), which provides a linker layer to the metal surface and the next layer. On the SAM a matrix of non-cross-linked carboxymethylated dextran is bound which provides a hydrophilic layer. The dextran is a linear chain of 1,6-linked glucose units and it exhibits a very low non-specific adsorption of biomolecules. Each glucose unit is modified with one carboxyl group (1). In most cases, the dextran hydrogel is the starting point to covalently bind a ligand to the sensor surface by amine-, thiol- or aldehyde chemistry.
The glass slide is made of a glass with a matching refractive index for the application chosen. Generally, this is standard BK7 glass (refractive index 1.26–1.38). However, for applications involving organic solvents other types like N-BAF3 (1.32–1.44) or NSF15 (1.40–1.52) can be used. Recently a fused silica prism was used which had a sensitivity 1.6 times higher than the BK7 glass prism at the same incident angle of 46.2° (2).
On one side of the glass, a linker layer is deposited (e.g., 2 nm Cr) and on top a layer of gold (48–50 nm). The gold layer will induce the surface plasmon resonance and is a good starting point for further modification of the sensor. It is possible to adsorb organic molecules like antibodies to the gold surface but many molecules undergo denaturation upon contact with a metal layer. Other molecules are too difficult to adsorb (3).
A thiol compound and a gold surface is one of the well-established combinations used when making a SAM (4). The n-alkanethiols (HS-(CH2)n-CH3, n > 10) is the most frequently used compound in producing SAM surfaces. The sulphur head group generally binds as a thiolate at the three-fold hollow site at the AU (111) crystal lattice. A slight mismatch between the pinning distance and the van de Waals diameter of the alkyl chain forces the molecules to assemble in a slightly tilted configuration in order to optimize the lateral interactions (5).
The SAM is easily made by dipping a gold surface in the alkanethiol solution. By using mixtures of modified alkanethiols several different SAM surfaces can be made (6). The hydrophilic layer serves partly as a barrier to prevent adsorption of proteins and partly as a starting point for further modification of the sensor (7).
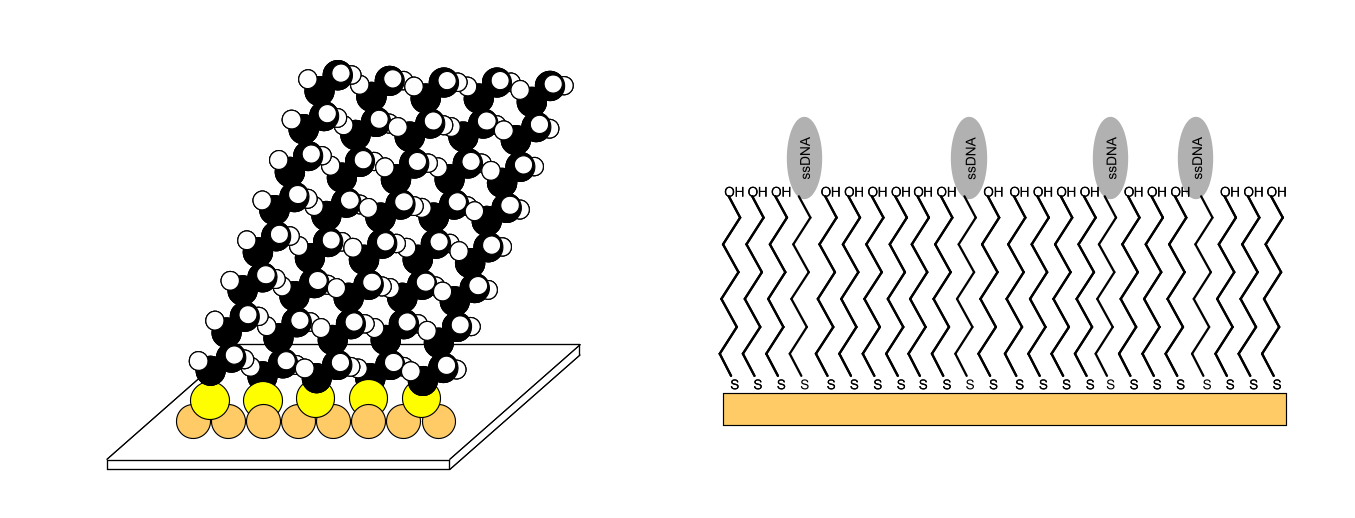
One of the common functionalities of the sensor surface is the attachment of a dextran layer. After making the SAM reactive, the dextran (500000 Da) is allowed to react with the SAM, resulting in a uniform layer of highly flexible and uncrossed linked dextran (1-3 ng mm-2) with an open structure that is approximately 100 nm thick. The reaction of the dextran with bromoaceticacid acid will result in approximately one carboxyl group per glucose unit. Although many illustrations depict the dextran layer as strands lying side by side, the real configuration of the dextran is a tangled structure.
The carboxyl group can serve as a starting point to attach a ligand to the sensor surface. With proper reaction conditions the negatively charged dextran matrix will attract a positively charged ligand, resulting in high local ligand concentrations and an efficient covalent reaction (7). The dextran layer is an effective barrier between the gold-SAM layer and the biomolecules, reducing non-specific adsorption and denaturation of the biomolecules. Furthermore, the dextran layer increases the capacity of binding sites for the ligand. The hydrogel binding capacity for proteins, expressed as surface concentration, is approximately 50 ng/mm3(8).
The prism-based SPR systems with disposable sensor chips use an optical glue (bis 2-hydroxyethylsulfide) to ensure maximum light coupling between prism and sensor chip. The chip, glass and glue have an index of refraction of 1.52.
For more in-depth information about sensor chips see the special sensor chip chapter.
References
| (1) | Biacore AB BIACORE Technology Handbook. (1998). |
| (2) | Yuk, J. S., D. G. Hong, J. W. Jung, et al. Sensitivity enhancement of spectral surface plasmon resonance biosensors for the analysis of protein arrays. Eur.Biophys.J. 35: 469-476; (2006). |
| (3) | Lofas, S., M. Malmqvist, I. Ronnberg, et al. Bioanalysis with surface plasmon resonance. Sensors and Actuators B 5: 79-84; (1991). |
| (4) | Wink, T., S. J. van Zuilen, A. Bult, et al. Self-assembled monolayers for biosensors. Analyst 122: 43R-50R; (1997). |
| (5) | Nagata, K. and H. Handa Rela-Time Analysis of Biomelecular Interactions. (2000). |
| (6) | Dojindo Molecular, T. Self-Assembled Alkenetthiol Monolayers. (2003). Goto reference |
| (7) | Lofas, S. and B. Johnsson A novel hydrogel matrix on gold surfaces in surface plasmon resonance sonsors for fast en efficient covalent immobilization of ligands. J.chem.soc., chem commun. 1526-1528; (1990). |
| (8) | Johnsson, B., S. Lofas and G. Lindquist Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Analytical Biochemistry 198: 268-277; (1991). |