These are the posts of the old forum. It was not possible to transfer the user data, so they are missing in most of the posts. For new questions, go to the general discussions.
1:1 / 2:1 stoichiometry
- OldForum
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
12 years 10 months ago - 12 years 2 months ago #1
by OldForum
1:1 / 2:1 stoichiometry was created by OldForum
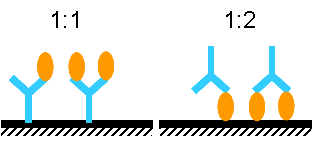
Hello. I would like clarification on stoichiometry and how it applies to protein molecular weights. See attached pic.
So a 1:1 binding is shown on the left and a 2:1 binding is shown on the right. Correct?
Would a 2:1 be more applicable for low molecular weight proteins, like 6,000? And a 1:1 would be more likely for higher molecular weight proteins, like 80,000? My thought is that the low mol. wt. would be smaller and they have more chances of two of them binding onto the 2 Ab binding sites, less crowding. The larger proteins would be larger and that it would prevent another protein of the same size binding to the other Ab site. Kindly comment. Thanks.
mtarca
So a 1:1 binding is shown on the left and a 2:1 binding is shown on the right. Correct?
Would a 2:1 be more applicable for low molecular weight proteins, like 6,000? And a 1:1 would be more likely for higher molecular weight proteins, like 80,000? My thought is that the low mol. wt. would be smaller and they have more chances of two of them binding onto the 2 Ab binding sites, less crowding. The larger proteins would be larger and that it would prevent another protein of the same size binding to the other Ab site. Kindly comment. Thanks.
mtarca
Last edit: 12 years 2 months ago by OldForum.
Please Log in or Create an account to join the conversation.
- OldForum
- Topic Author
- New Member
-

Less
More
- Thank you received: 0
12 years 10 months ago - 10 years 11 months ago #2
by OldForum
Replied by OldForum on topic 1:1 / 2:1 stoichiometry
Hi,
In SPR the ligand is of no importance in the stoichiometry. Although the density of the ligand can have an effect on the stoichiometry of for instance antibodies. If you have a low ligand concentration, the antibody interaction will be predominantly 1:1. When you have a high ligand concentration it will be shifted to the 1:2 (bivalent analyte model). If a bivalent model is applicable is not determined on the basis of the ligand or analyte size but by the interaction sites. You are right that if the first interaction is occluding the second interaction site there will be mixed interactions which are more difficult to solve. Therefore it is better to immobilize the multivalent interactant when possible.
What does it do for the kinetics? The 1:2 interaction benefits from two interactions holding one analyte in place. So the apparent interaction looks stronger (lower dissociation rate). To resolve the single interaction kinetics, either use a low ligand density or immobilize the multivalent interactant.
I hope this will explain the stoichiometry in SPR.
The model is explained at www.sprpages.nl/data-fitting/models/bivalent-analyte.html
Kind regards
Arnoud
In SPR the ligand is of no importance in the stoichiometry. Although the density of the ligand can have an effect on the stoichiometry of for instance antibodies. If you have a low ligand concentration, the antibody interaction will be predominantly 1:1. When you have a high ligand concentration it will be shifted to the 1:2 (bivalent analyte model). If a bivalent model is applicable is not determined on the basis of the ligand or analyte size but by the interaction sites. You are right that if the first interaction is occluding the second interaction site there will be mixed interactions which are more difficult to solve. Therefore it is better to immobilize the multivalent interactant when possible.
What does it do for the kinetics? The 1:2 interaction benefits from two interactions holding one analyte in place. So the apparent interaction looks stronger (lower dissociation rate). To resolve the single interaction kinetics, either use a low ligand density or immobilize the multivalent interactant.
I hope this will explain the stoichiometry in SPR.
The model is explained at www.sprpages.nl/data-fitting/models/bivalent-analyte.html
Kind regards
Arnoud
Last edit: 10 years 11 months ago by .
Please Log in or Create an account to join the conversation.